
8 | 9
CAMLOG
®
Implant Position Planning
_
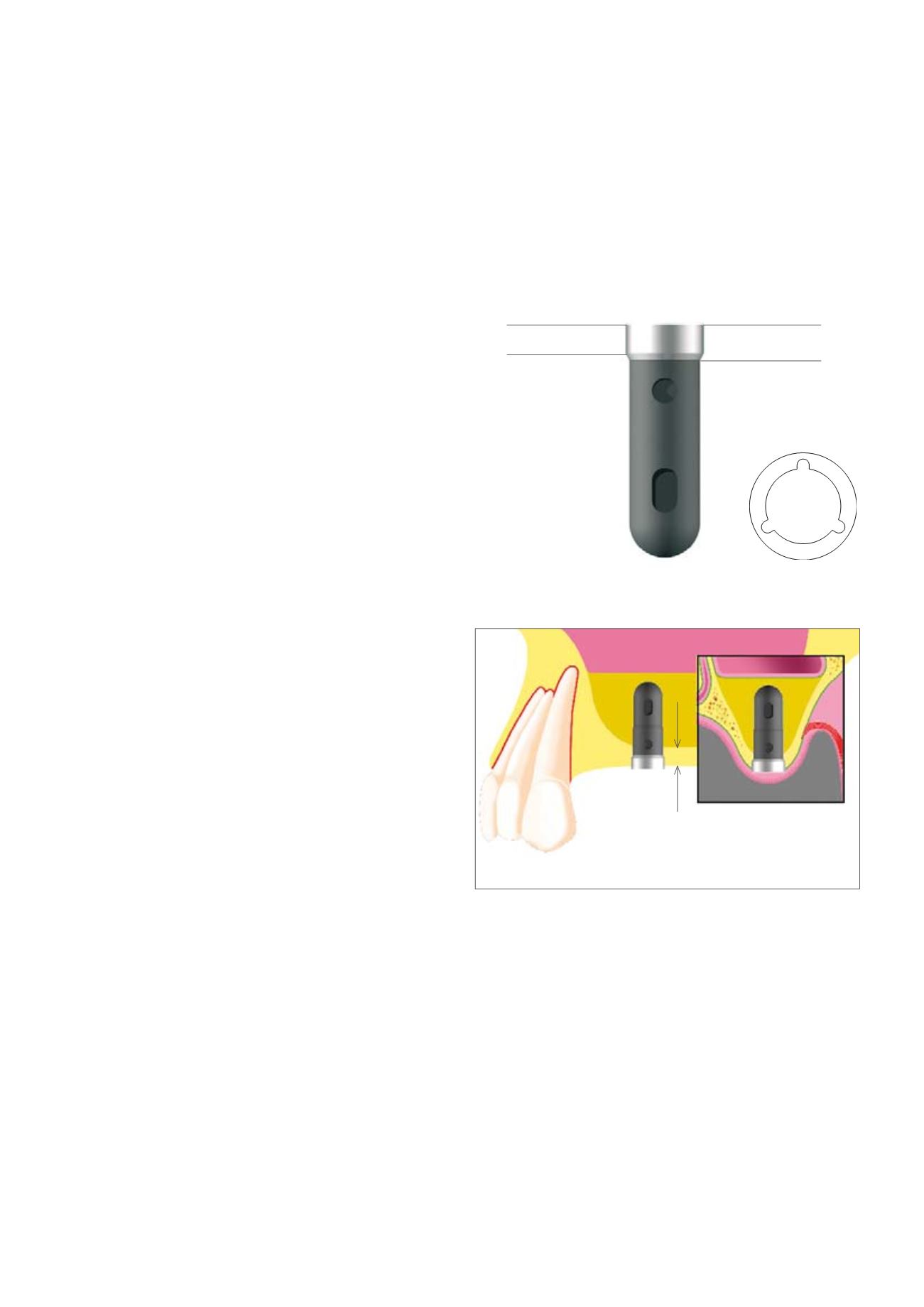
2.0 mm
CYLINDER-LINE IMPLANTS
CYLINDER-LINE implants represent cylinder implants in their geometry and
have a titanium plasma coating (TPS), (2.0 mmmachined implant neck part).
A particular advantage of this implant type is its simple, time-saving appli-
cation procedure. In conjunction with sinus floor elevation and augmenta-
tion, the press-fit from the cylindrical configuration of the implant allows it
to be placed in a structurally weak site with a residual bone height < 5 mm.
The Tube-in-Tube™ inner configuration has not been changed.
RECOMMENDED INDICATIONS
s¬ %NDOSSEOUS¬USE¬IN¬THE¬MAXILLA¬AND¬MANDIBLE¬FOR¬FUNCTIONAL¬ESTHETIC¬REHA
-
bilitation in partial or fully edentulous patients
s¬ 5SE¬IN¬STRUCTURALLY¬WEAK¬SITES¬IN¬THE¬POSTERIOR¬MAXILLA¬IN¬CONJUNCTION¬WITH¬
sinus floor elevation and augmentation.
CYLINDER-LINE implants are mounted in the sterile packaging with an in-
sertion post color-coded corresponding to their diameter.
IMPLANT DIAMETERS:
3.3/3.8/4.3/5.0/6.0 mm.
IMPLANT LENGTHS:
9/11/13/16 mm
(9 mm length not available for implant diameter 3.3 mm)
INDICATIONS FOR CAMLOG
®
IMPLANTS
WITH 3.3 MM DIAMETER
Implants with 3.3 mm diameter can only be used for the mandibular inci-
sors and lateral maxillary incisors. Telescopic crown structures on implants
with 3.3 mm diameter are not allowed. An edentulous arch can be restored
with a prosthesis only if this is bar-splinted with four implants of 3.3 mm
diameter without distal extensions. 3.3 mm implants are suitable for a par-
tially edentulous arch when combined with implants of larger diameters for
splinted superstructures. Avoid excessive mechanical stressing of the im-
plants when using bar and ball abutments with 3.3 mm implants. The use
of Locator
®
abutments for implant divergences of greater than 10° per im-
plant is contra-indicated.
TPS
1.6 mm
< 5 mm


















